(Disclaimer: Names and identifying details have been changed to protect the privacy of the individuals included in the article.)
By March 1, the Institute for Health Metrics and Evaluation (IHME) projects that 438,941 Americans will die as a result of Covid-19. Due to this projection, drug companies are racing faster than ever towards finding a vaccine for the novel coronavirus. A few of them, at the moment, have even reached phase three of human clinical trials. And if you have seen the news lately, Pfizer and Moderna have been prime examples of companies already succeeding during this phase, with both reporting a 94.5%+ efficacy rate for their vaccines in their initial and final results. In fact, one senior here even participated in one of their human clinical trials. More specifically, Pfizer’s.
Meet Barron Goldblum:
After turning 18 in September, Barron Goldblum decided that he wanted to sign up for the Covid-19 vaccine human clinical trials. A decision he made carefully after contemplating the pros and cons for a few days. How he did this was through the COVID-19 Prevention Network (CoVPN) website, the clinical trial network established by the National Institute of Allergy and Infectious Diseases (NIAID) in July 2020. Through the website, he could submit a survey that would go into depth about his own physicality, family living situation, amongst other personal questions.
Once approved, Goldblum was then admitted into the Pfizer/BioNTech human clinical trial program, one of the first companies to begin human clinical trials. Even more, one of the first companies to administer an mRNA-based vaccine. A vaccine that Goldblum was ultimately inoculated with, twice.
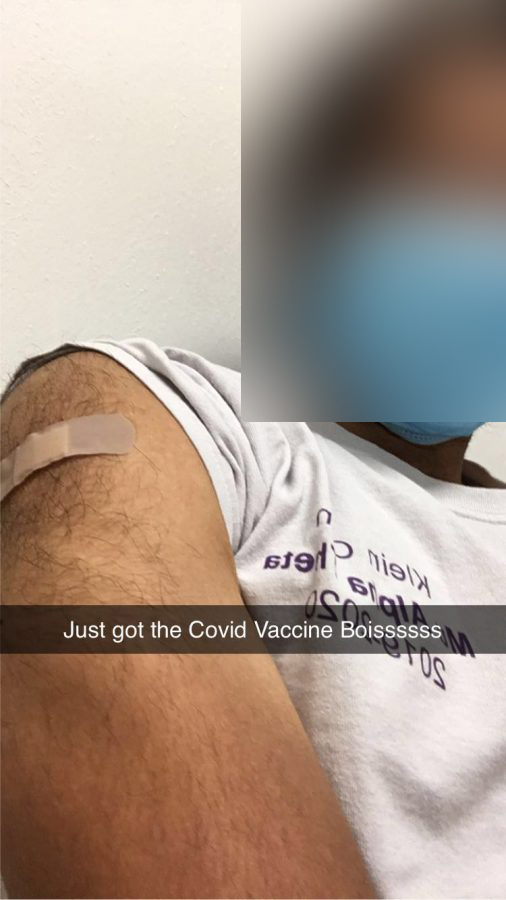
“They asked for my medical history if I’ve had any vaccines in the past two months, which I had not,” Goldblum said.
In the study, over 44,000 participants were either injected with the real mRNA-based vaccine or saline placebo (to ensure that the vaccine did work). And by accident, Goldblum discovered that he did indeed receive the real vaccine. A discovery he made while unintentionally peeking at the syringe his physician-administered.
“It was slightly orange. The color of the vaccine,” Goldblum recounted.
The vaccine, while promising prevention of Covid-19 from severely damaging Goldblum’s body, came with its own side effects. Just days after he received the shot, Goldblum reported swelling in the gums and severe pain at the injection site, both of which caused Goldblum to consult his dentist to see whether the problem was either due to the vaccine shot or dental problems. Ultimately, though, Goldblum’s dentist “confirmed that it was likely an adverse event from the vaccine shot”. After that frightening situation, however, Goldblum began to call his researchers more often through the 24-hour hotline given to him at the beginning of the trial.
“I called several times to ensure it was going smoothly but supposedly I was the only one with this adverse reaction,” said Goldblum.
As time progressed, Goldblum would detail any side effects of the virus and depict how he felt each week in a diary given to him by his researchers. Further, Goldblum would also visit his researchers every month to talk with them in-person about any potential problems not written about in his diary. Finally, just last week, Goldblum was given his final/second booster shot to increase his body’s immunity to the novel coronavirus.
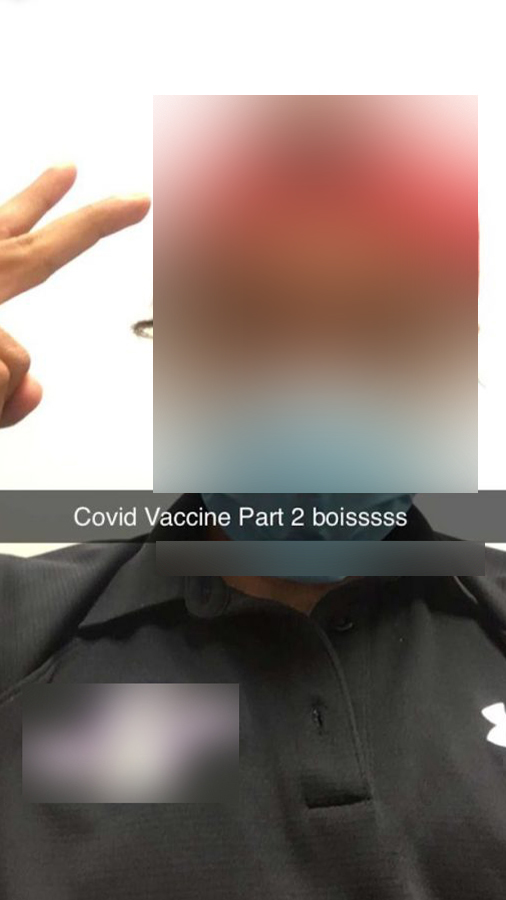
“The second visit was fine and I felt good for an hour or two after the visit,” Goldblum recalled. “However, [the booster vaccine] made me nauseous, gave me migraines, and [it] felt like my stomach was being pummeled. Also, I was delirious in my sleep the night I got the vaccine. After two days though I was back to normal.”
Ultimately, though, after receiving news that Pfizer’s vaccine had a 95% efficacy rate in addition to his $120 compensation for each visit, Goldblum felt great about choosing to volunteer for the human clinical trial. When asking senior Walter Night, one of Goldblum’s friends, about how he felt about Goldblum volunteering for the trial, Night remarked that it was “a good and brave thing for him to do”.
“[Personally], I did try to volunteer and signed up for it, but I was denied because I’m not 18,” said Night.
(To see the number of COVID-19 cases in Klein ISD, visit Klein ISD’s COVID-19 dashboard.)